Unlocking the Secrets of Alpelisib
Before diving into the specifics of how alpelisib works, it's crucial to understand what this drug is. Alpelisib is a medication used in the treatment of metastatic breast cancer, especially in postmenopausal women and men. This breakthrough drug has significantly impacted the cancer therapy field, offering hope to patients who previously had limited treatment options. As we progress in this article, we will further break down the mechanism of action of alpelisib in cancer therapy.
The Mechanism of Alpelisib
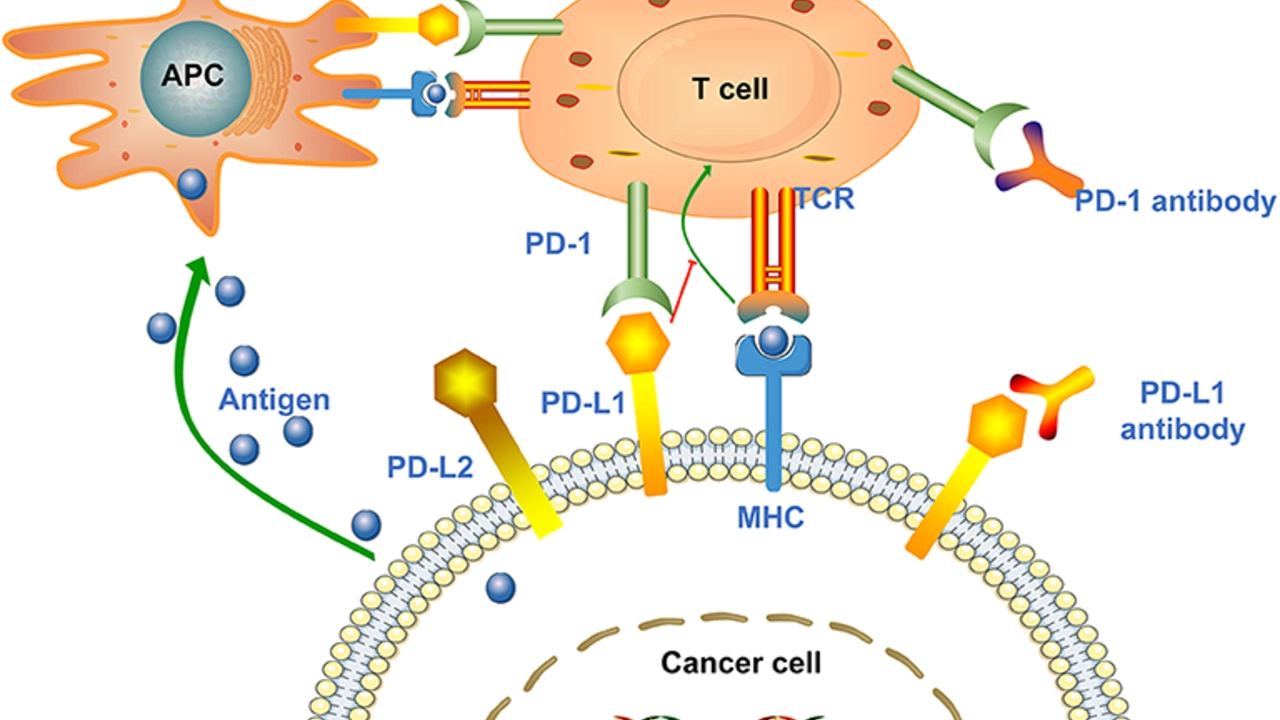
The precise mechanism of alpelisib is indeed fascinating. This drug functions by inhibiting the protein PI3K, which is often overactive in cancer cells. By blocking this protein, alpelisib can slow down or stop the growth of cancer cells. This mode of action makes it an effective tool in the fight against breast cancer, specifically for tumors that have a specific genetic mutation, known as PIK3CA.
Targeting the PIK3CA Mutation
The PIK3CA mutation plays a pivotal role in the development and progression of certain types of cancer. This mutation leads to an overactive PI3K protein, causing cells to grow and divide more rapidly than they should. Alpelisib specifically targets cancer cells with this mutation, providing a tailored approach to cancer therapy.
Alpelisib and Hormone Therapy
Alpelisib does not work alone. It is used in combination with a type of hormone therapy called fulvestrant. This combination treatment has been shown to be more effective than hormone therapy alone. Fulvestrant works by blocking estrogen receptors, which breast cancer cells often depend on for growth. When used together, alpelisib and fulvestrant can deliver a one-two punch to cancer cells, slowing their growth and potentially leading to their destruction.
Understanding the Clinical Trials
Like all new drugs, alpelisib went through rigorous clinical trials before it was approved for use. These trials have shown promising results. In one study, patients treated with alpelisib and fulvestrant had a significantly longer progression-free survival than those treated with fulvestrant alone. We'll delve into these trials in detail, discussing their design, results, and implications for future cancer treatment.
Side Effects of Alpelisib
While alpelisib has shown great promise in treating certain types of breast cancer, it's important to understand that like all medications, it can cause side effects. These can include nausea, fatigue, and rash, among others. We'll provide comprehensive information about these potential side effects, as well as tips for managing them.
Future Applications of Alpelisib
As we continue to understand more about the mechanism of action of alpelisib, the future applications of this drug are exciting. Although currently approved for use in breast cancer, there's potential for alpelisib to be used in other types of cancers that have the PIK3CA mutation. We will explore these potential future applications and the ongoing research in this area.
Living with Cancer: Patient Experiences with Alpelisib
To truly understand the impact of alpelisib on cancer treatment, it's essential to hear from those directly affected: the patients. In this section, we'll share stories from patients who have been treated with alpelisib, shedding light on their experiences, their struggles, and their victories.

Comments
Cassidy Strong
While the article offers a broad overview, it regrettably omits critical details regarding the pharmacokinetic profile of alpelisib; moreover, the discussion of adverse events lacks statistical granularity, which is indispensable for clinicians. The mechanistic explanation, though accurate, could benefit from a deeper exploration of PI3K isoform selectivity, especially in the context of resistance mechanisms. Additionally, citing the exact hazard ratios from the SOLAR-1 trial would bolster the credibility of the efficacy claims. Finally, a brief comparison with other PI3K inhibitors would provide a more balanced perspective.
July 26, 2023 at 16:48
Anil Karwal
Interesting points raised, especially about the need for more granular side‑effect data. I think the article does a decent job of summarizing the combo with fulvestrant, though a bit more on patient quality‑of‑life would be nice.
August 28, 2023 at 02:24
Suresh Pothuri
Alpelisib's efficacy is proven, and the data speak for themselves.
September 29, 2023 at 12:00
Millsaps Mcquiston
It works because it hits PI3K directly, stopping tumor growth. Simple as that.
October 31, 2023 at 20:36
michael klinger
One cannot overlook the broader implications of introducing a targeted agent such as alpelisib into the therapeutic arsenal; the pharmaceutical lobby has, quite evidently, orchestrated a narrative that emphasizes novelty while downplaying long‑term safety concerns. The regulatory approval process, shrouded in opaque deliberations, raises questions about the extent to which independent data were truly considered. Moreover, the partnership with hormone therapy appears to be a strategic move designed to maximize market penetration under the guise of clinical benefit.
December 3, 2023 at 06:12
Matt Laferty
To fully appreciate alpelisib's role in contemporary oncology, one must first recognize the complex interplay between the PI3K/AKT/mTOR pathway and hormone‑driven tumorigenesis; this pathway, when hyperactivated by PIK3CA mutations, drives relentless cellular proliferation and survival, rendering conventional endocrine therapies insufficient.
Alpelisib, as a selective PI3Kα inhibitor, directly curtails this aberrant signaling, thereby restoring sensitivity to endocrine agents such as fulvestrant.
Clinically, the pivotal SOLAR‑1 trial demonstrated a median progression‑free survival extension of approximately 7.5 months in patients harboring PIK3CA mutations, a gain that is statistically and therapeutically meaningful.
Importantly, the trial also highlighted an improved overall response rate, underscoring the drug's capacity to elicit tangible tumor shrinkage.
Nevertheless, the toxicity profile cannot be dismissed; hyperglycemia, rash, and diarrhea emerged as the most frequent adverse events, necessitating proactive monitoring and dose adjustments.
Management strategies, including antidiabetic agents for glucose dysregulation and topical steroids for dermatologic reactions, have become integral to therapeutic protocols.
The combination with fulvestrant is not merely additive but synergistic, as fulvestrant degrades estrogen receptors while alpelisib blocks downstream proliferative cues, delivering a one‑two punch against cancer cells.
Real‑world evidence from post‑approval registries corroborates the trial data, showing consistent efficacy across diverse patient populations, albeit with variable adherence due to side‑effect burden.
Furthermore, emerging data suggest potential utility of alpelisib beyond breast cancer, with early‑phase studies exploring its activity in other PIK3CA‑mutated malignancies such as endometrial and ovarian cancers.
These investigations are paving the way for broader indications, contingent upon careful patient selection and biomarker validation.
From a pharmacoeconomic standpoint, the cost‑effectiveness of alpelisib remains under scrutiny; while the drug offers clinical benefit, its high price poses access challenges, especially in healthcare systems with limited resources.
Health‑technology assessments are ongoing to determine reimbursement frameworks that balance innovation with sustainability.
In summary, alpelisib represents a significant advancement in precision oncology, delivering meaningful benefits to a genetically defined subset of breast cancer patients.
Its success underscores the importance of molecular profiling, interdisciplinary management of side effects, and continual research to expand its therapeutic horizon.
January 4, 2024 at 15:48
Write a comment